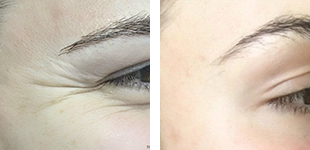
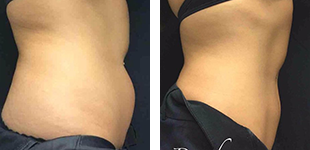
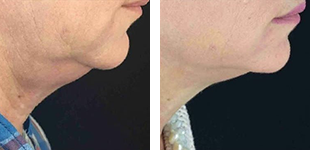
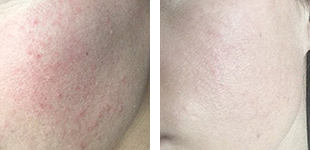
One of the most important aspects when purchasing an aesthetic devices is they meet the appropriate standards and approval from Food and Drug Administration regulations to ensure the device is safe and effective for it’s intended use. However, not all imported laser manufactures obtain FDA clearance for their devices. Let’s dive in to the world of lasers!
The US aesthetic device market is experiencing a shakeup with the rise of Chinese manufacturers, who are offering competitively priced, technologically advanced devices. This influx has challenged established manufacturers, creating intense price competition and pressuring them to innovate and lower profit margins. The affordability of Chinese devices has also made advanced treatments more accessible for smaller clinics, broadening patient options.
However, the entry of these devices raises concerns. Some Chinese products lack FDA approval, posing potential risks to patient safety and creating legal challenges for practitioners. Knock-off brands that mimic US devices without meeting safety standards have also emerged, emphasizing the need for careful verification.
For practitioners, the benefits of affordable options come with the responsibility to confirm FDA clearance and avoid counterfeits to ensure patient safety. As the market continues to evolve, balancing cost savings with regulatory compliance and quality assurance will be essential for success in the industry.
 DermFx
DermFx